Redox flow technology
The term redox is a short word made up of 'red' (for reduction) and 'ox (for oxidation). It describes a chemical reaction in which electrons are given off (oxidation) and taken up by a reaction partner (reduction). During this process, electrical energy is released or chemically bound.
Research on this began in the middle of the 20th century. In 1986, Maria Skyllas-Kazacos and her team at the University of New South Wales patented the first electrochemical energy storage device, the vanadium redox flow battery.
The technology is based on the storage of electrical energy resulting from the potential difference when two redox pairs react with each other. The main difference to other battery technologies - apart from the energy density - is that the reaction partners of the storage medium are in dissolved form as electrolytes in tanks. In addition to vanadium-sulfuric acid as the electrolyte, there are now a number of differently structured electrolytes on a metallic and organic basis.
Design and operation of a redox flow battery
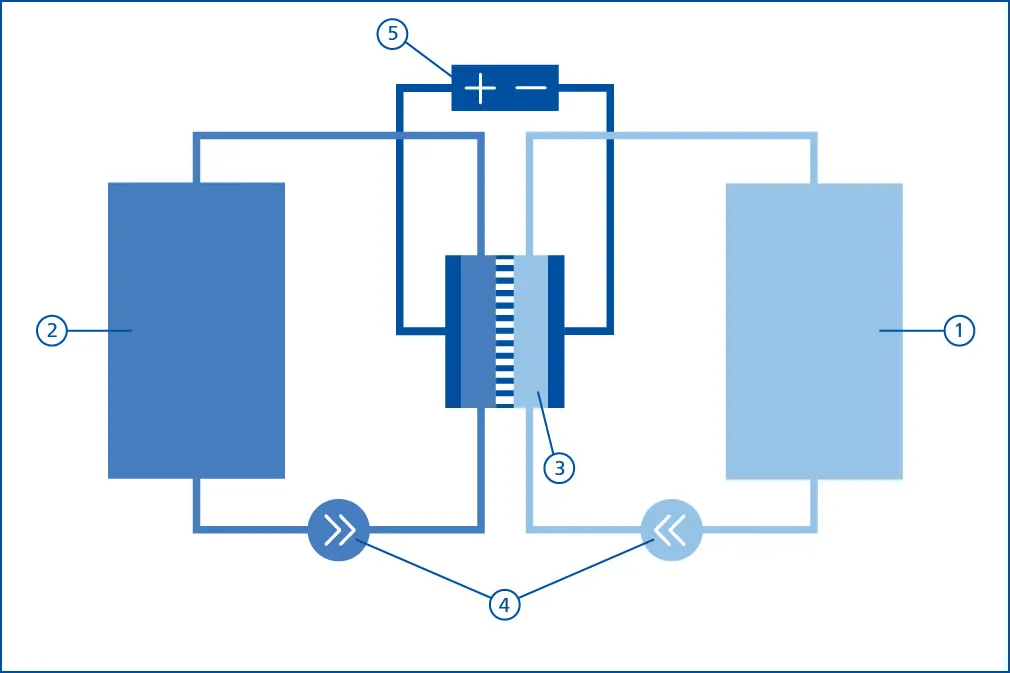
A redox flow battery consists of 5 main components:
| Number | Main component |
|---|---|
| 1 | Anolyte tank |
| 2 | Catholyte tank |
| 3 | Electrochemical converters (stack) |
| 4 | Fluid system incl. pumps, sensor technology and actuator |
| 5 | Power electronics incl. grid connection |
Each redox flow battery uses two tanks in which the electrolytes are stored. One tank contains the negatively charged electrolyte (catholyte), the other tank contains the positively charged electrolyte (anolyte).
The stack is constructed from a defi ned number of individual battery cells, with each half-cell divided by an ion-exchange membrane. The battery cells are each separated by a fluid-tight, conductive bipolar plate. The actual electrochemical reaction takes place in the graphite felts of the half cells, through which the respective positive or negative electrolyte flows. To charge or discharge the battery, the electrolyte is pumped through the stack. As soon as an electrical load is applied to the stack, the electrochemical reaction begins: the current flows and the charge is removed from the electrolyte. To recharge the electrolytes, an external voltage is applied so that the reaction is reversed.
The energy density of the battery depends on the respective electrolyte. In the most commercially widespread variant of vanadium redox flow technology, it is 15 to 20 Wh/liter in practice.
FAQ
1. Can you buy complete, ready-to-connect batteries from Schmalz?
No. Schmalz is a manufacturer of components and subsystems. Schmalz develops and produces the power component (stack) of a redox flow battery and equips it with peripherals (sensors, etc.) if required. Schmalz stacks are used by system integrators in their complete systems.
2. Does the stack store power?
No. The stack is responsible for charging and discharging a liquid (electrolyte). The electrical energy is stored chemically in the liquid. The stack is therefore the electrochemical converter of a redox flow battery.
3. Can a stack be operated with other electrolytes?
In principle, yes. However, modifications are often necessary. It must always be checked in advance to what extent the electrolyte matches the components in the stack.
4. Are there stacks with different performance classes?
Yes. The performance of a stack results from the number of cells. This can be adjusted individually.
5. Is the number of cells within a stack limited?
In practice, the number of cells in a stack is limited by electrochemical processes - depending on the electrolyte used.
6. Can the fluid connections be modified?
Yes. Schmalz uses standardized connections that can be adapted if necessary.